Periodic table
The periodic table is a method of classifying elements and its use to predict properties of elements.
Periodic trends
Groups
Elements are arranged in order of proton number. Elements with similar chemical properties are placed in the same vertical column called groups. Elements in a group have similar chemical properties, same outer electron numbers, and usually the same valency. Going down the group, the elements become more metallic in character.
Periods
The horizontal rows are called periods. Moving across a period, the elements change from metallic to non-metallic. The number of valency electrons increases across the period but the number of occupied energy levels (i.e. shells) stays the same.
Metals vs non-metals
The main physical differences are summarized in this table:
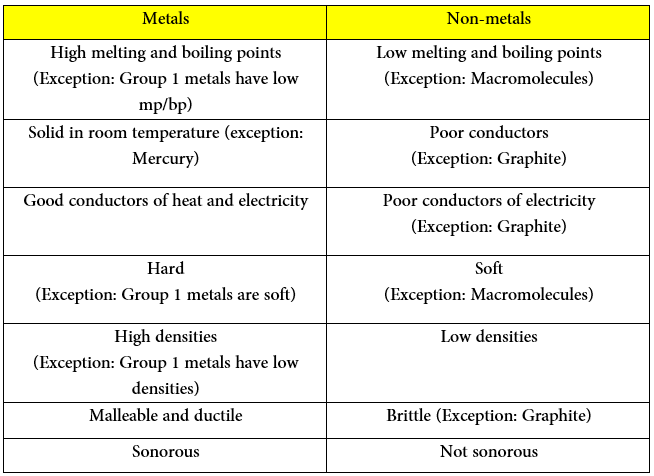
The main chemical differences are summarized in this table:
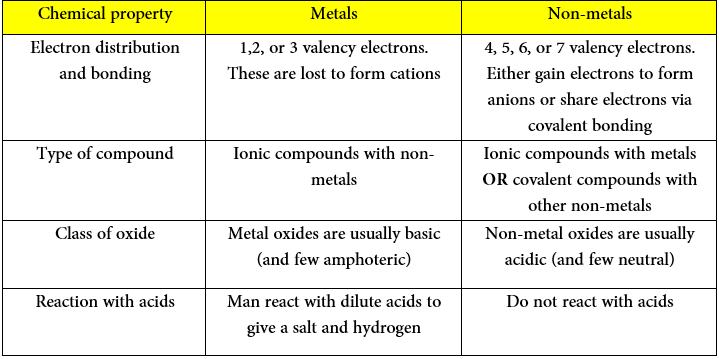
*Many of this material has been covered in previous topics. For more information about chemical bonds click here. For more information about acids and bases click here.
Group properties
Group 1 – Alkali metals (i.e. Li, Na, K)
These are extremely reactive metals. The alkali metals (despite being metallic) are rather soft and have low m.p/b.p compared to other metals. They are good conductors of heat and electricity and demonstrate shiny surfaces when freshly cut.
Due to their low valency, these metals are extremely reactive. Each element will react with cold water to form a hydroxide and hydrogen.
- Physical trends down the group
- Increasing softness
- Decreasing melting/boiling points
- Increasing densities
- Chemical trends down the group
- Increasing reactivity
- Lithium reacts steadily with water whereas potassium may cause an explosion
- Increasing reactivity
Group 7 – Halogens (i.e. Cl, Br, I)
Halogens are a collection of diatomic non-metals showing both physical and chemical trends down the group.
- Physical trends down the group
- Colour gets darker down the group
- Chlorine is yellow/green
- Bromine is brown
- Iodine is a black solid with purple vapour
- M.p and b.p increase down the group
- Chlorine is a gas (rtp)
- Bromine is a liquid (rtp)
- Iodine is a solid (rtp)
- Colour gets darker down the group
- Chemical trends down the group
- Decreasing reactivity
- Chlorine can displace both bromine and iodine from their compounds:
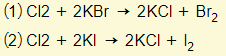
- Chlorine can displace both bromine and iodine from their compounds:
- Decreasing reactivity
*Don’t be too confused about the concept of ‘displacement’. All that it means is that chlorine is more reactive than both bromine and iodine. Therefore, in reaction (1), chlorine has the ability to ‘kick out’ the bromide ion from the compound and take its place. Chlorine will therefore become an ion to form an ionic bond with potassium (KCl), whereas bromine becomes a molecule (gas).
Transition elements
These are metallic elements placed in the middle of the periodic table.
- Physics properties
- Compared to groups I and II, they have higher densities and melting points. They are also harder and stronger.
- Chemical properties
- Compared to groups I and II, they are a lot less reactive
- They do not react with cold water but many react when heated in steam:
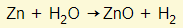
- They have more than one valency. Iron (Fe), for example, forms two different types of ions:
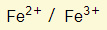
- This means that they can either decide to lose 2 or 3 electrons to become an ion
- They form coloured compounds
- Iron (II) salts are pale green whereas iron (III) salts are yellow/brown
- Copper (II) salts are blue
- Nickel salts are bright green
- They do not react with cold water but many react when heated in steam:
- Compared to groups I and II, they are a lot less reactive
*You are NOT required to know the specifics of why transition elements have multiple valencies (or oxidation states) whereas other elements do not
Group 0 – Noble gases
Noble gases are unreactive; they have a valency of 0. Their outer electron shell is already complete.
Noble gases have various uses:
- Helium is used in balloons. The balloon will float because helium is less dense than air and also safer (because they cannot catch fire due to their unreactiveness)
- Argon is used to fill electric light bulbs because it is inert