States of matter
Matter is any substance that occupies physical space.
The kinetic theory of matter states that matter is made of tiny particles (i.e. atoms and molecules) and that they are always in constant motion.
There are three states of matter that you need to be aware of: Solids, liquids, and gases.
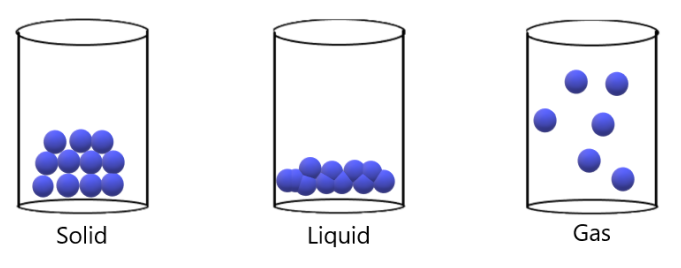
Solids have particles that are packed closely together. The atoms are arranged in a regular pattern due to the strong attractive forces that exists between the particles. These particles therefore cannot move. Instead, they vibrate constantly on the spot.
By giving a solid energy (i.e. heating), the particles will begin to vibrate more vigorously as they gain more energy. Eventually, the particles begin to separate as they start to overcome attractive bonds. Eventually, the particles will have enough separation to move past one another, but still kept relatively close (as attractive forces between particles still exist). The solid has now officially become a liquid.
Liquids therefore have particles that are loosely arranged (i.e. can move past one another). They therefore take up the shape of the container in which they’re in. The particles themselves are constantly moving in constant, random motion.
By further heating the liquid, the particles gain even more energy! This will separate the particles even more as they overcome most of the remaining forces of attraction that exists.
Gases therefore have particles that are very far apart. Again, the particles will be moving in constant, random motion and also take up the shape of its container. Unlike solids and liquids, gases can be compressed.
Bear in mind that if you start to take energy away from a gas (i.e. cooling) then individual particles will have less energy to overcome attractive forces, and will eventually turn back into a liquid. Similarly, by cooling a liquid, it will turn into a solid.
Here is a diagrammatic summary of the above:
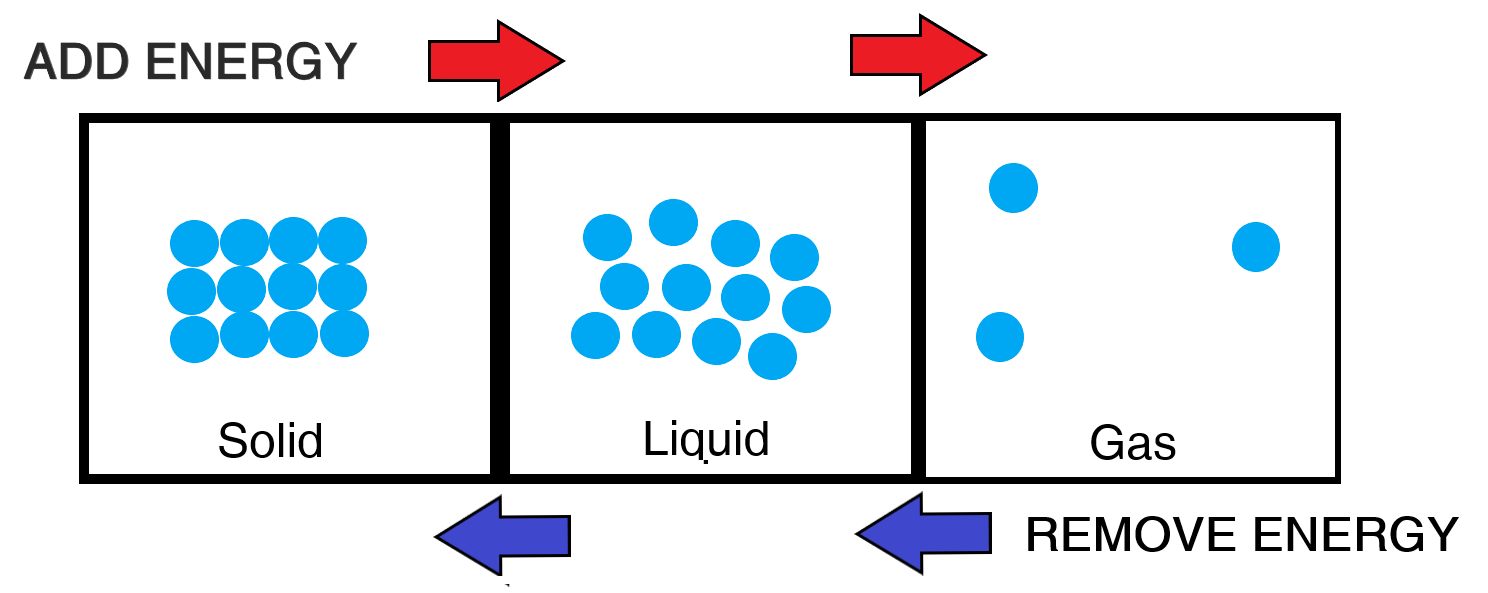
Transitioning from one state of matter to another
There is a name for each of the respective changes from one state to another. The diagram below is a good summary of these:
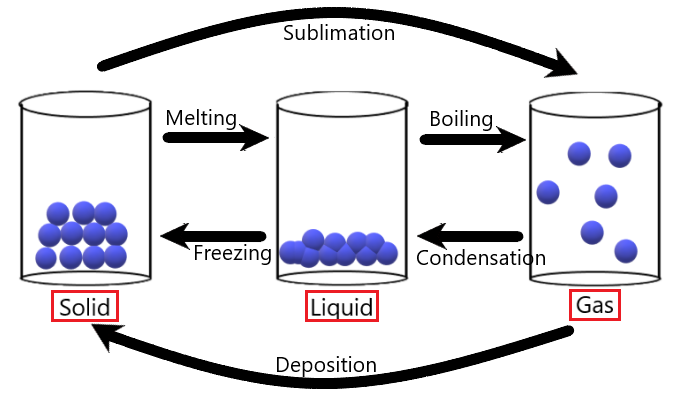
* A change from liquid to gas can also be called evaporation. However evaporation and boiling are two very different things. These two terms will be differentiated in detail in another topic. Just memorize boiling for now.
Brownian motion
One day, a scientist was observing a pollen grain suspended in water. He realized that the pollen grain was actually constantly moving in random directions. It looked like this:
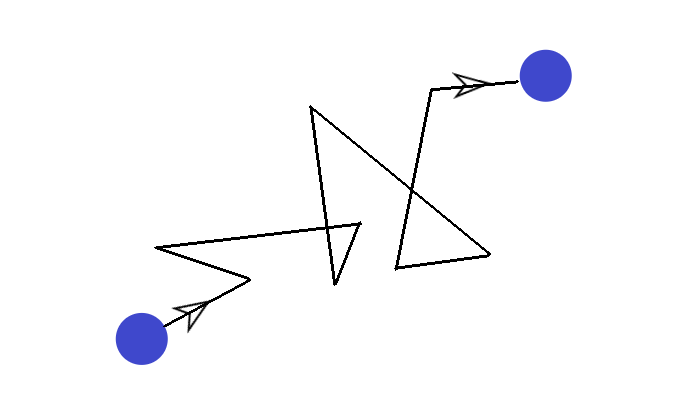
This made the scientist wonder why the pollen grain was moving in this fashion… And he figured it out.
Remember the kinetic theory of matter? Particles are always in constant motion. This meant that the water molecules were actually bombarding the pollen grain in random directions to cause the sort of movement observed above.
Brownian motion is therefore defined as the erratic random movement of microscopic particles in a fluid (i.e. pollen), as a result of continuous bombardment from molecules of the fluid (i.e. water molecules).
Diffusion
Diffusion is the net movement of particles from a region of their higher concentration to a region of their lower concentration down a concentration gradient.
The constant random movement of particles (and their kinetic energy) allows diffusion to occur. Ultimately this means that particles will spread out from one place to another.
There are many things that can affect the rate of diffusion. Molecular mass is one of these things. Heavier molecules will travel slower than lighter molecules. In other words, the higher the molecular mass, the slower the rate of diffusion.