The structure of an atom
Elements are substances that are made of a single type of atom.
Every atom has a central nucleus which contains smaller sub-particles called protons and neutrons. These sub-particles make up most of the weight of the entire atom.
- Protons are positively charged
- Neutrons have no charge
Electrons are much extremely small particles that orbit the nucleus of an atom.
- Electrons are negatively charged
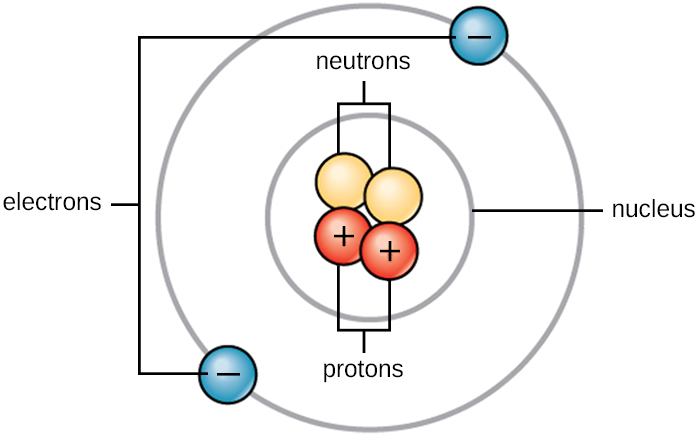
In a neutral atom (no surplass charge) the number of electrons orbiting the nucleus is equal to the number of protons inside the nucleus. In other words, the positive charge of the protons are canceled by the negative charge of the electrons.
Atoms can become charged by losing or gaining electrons. When this happens they are called ions not atoms.
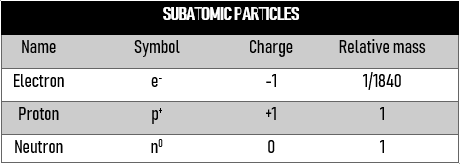
This table summarizes the sub-atomic particles within an atom, and their relative charges and weights.
What makes an atom unique?
An element is a substance made of a single type of atom. For example, the element carbon will only contain carbon atoms. The element oxygen will only contain oxygen atoms.
So what makes carbon different from oxygen? In other words, what makes a carbon atom different from an oxygen atom?
The atoms of an element all have a unique number of protons in their nucleus. This also means that each atom has a unique number of electrons (since proton number = electron number).
For example:
- Carbon atoms will always have 12 protons and therefore 12 electrons
- Oxygen atoms will always have 8 protons and therefore 8 electrons
The elements carbon and oxygen behave completely differently because their atoms have have completely different protons/electron numbers.
Isotopes
Whilst every atom of an element i.e carbon will have the same number of protons (12), they can have varying numbers of neutrons.
Atoms of the same element with varying neutron numbers (and therefore nucleon numbers) are called isotopes.
A nucleon number is the sum of protons and neutrons in an atom. It signifies the atoms mass since electrons are basically weightless.
For example, some carbon atoms (carbon-12) have 6 neutrons in its nucleus. Other carbon atoms (carbon-14) have 8 neutrons instead. Whilst the proton number is exactly the same (6 protons), the differences in neutron numbers will change the overall mass of the atom.
- Carbon-12 is the most common, and has a stable nucleus with the protons and neutrons being held firmly
- Carbon-14 however is unstable, and is radioactive
Nuclear fission & fusion
Two nuclei can interact by either fusing or breaking apart into small pieces:
- Nuclear fusion is the process by which two light nuclei combine together to release vast amounts of energy.
- Nuclear fusion is when an unstable, heavy nucleus splits into two lighter nuclei.
Nuclide
For any given atom:
- The proton number (Z) is the number of protons in the nucleus
- The nucleon number (A) is the number of protons and neutrons in the nucleus
A nuclide is an atom which is specified by the proton number and the nucleon number in the form of:
This means that as long as you have the nucleon number and the proton number, you can find out the number of neutrons in an atom since proton+neutron=A
So if atom A had 14 protons with a nucleon number of 20, then the no. of neutrons would be 6.
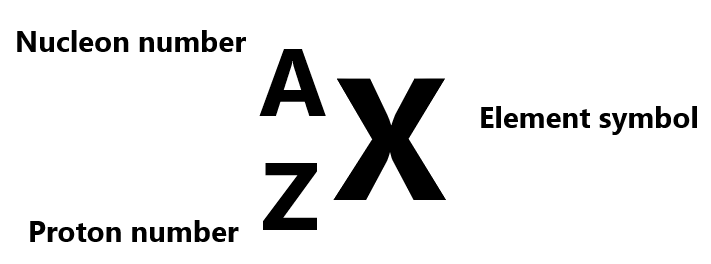
Discovery of the nucleus
Small positive particles (called alpha particles) were fired at a thin gold foil. The pathway that the particles took upon hitting the foil was observed and interpreted.
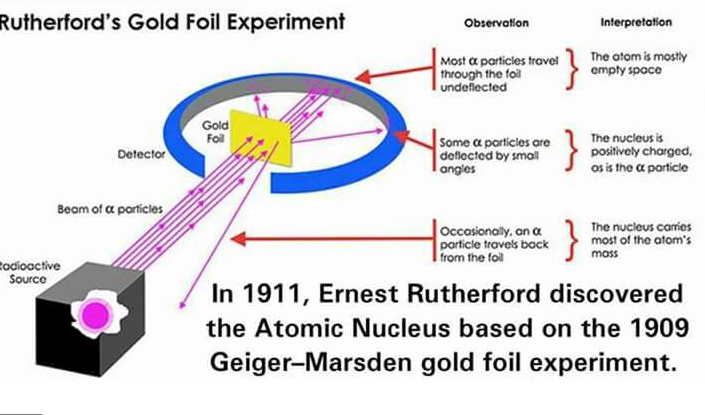
The following observations were made and interpreted
- Most of the particles traveled straight through the foil without changing direction.
- This suggested that most atoms within the foil were just empty space, and therefore the alpha particles went through without interacting with anything
- In other words, the nucleus is extremely tiny compared to the size of the entire atom. The alpha particles were much more likely to simply pass through the atom instead of interacting with the nucleus.
- Some particles were deflected slightly
- This suggested that the nucleus was positively charged, and therefore if an alpha particle did end up close to the atomic nucleus, it would be repelled by the positive nucleus and deflected to this side
- Rarely, particles would be reflected straight back from the foil (instead of passing through it)
- This suggested that the nucleus carried most of the atom’s mass. Therefore if any particles hit the nucleus, it would rebound right back.