Background
Organic chemistry is the branch of chemistry that deals with carbon compounds (other than simple salts such as carbonates, oxides, and carbides).
A homologous series is a family of similar compounds with similar chemical properties due to the presence of the same functional group.
A functional group is a set of atoms which dictate the chemical properties of that compound. Therefore, compounds that share that same functional group will inherently share similar chemical properties.
The table below demonstrates the names of all the different functional groups that exist in organic chemistry.
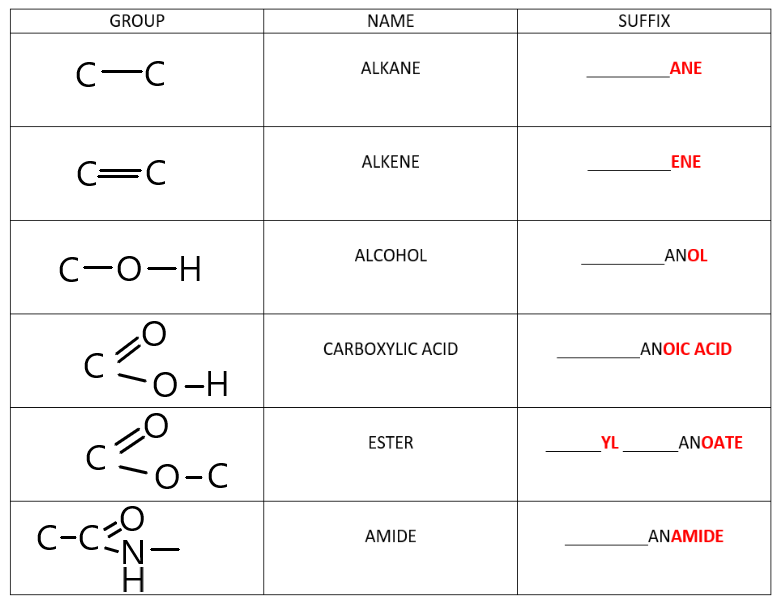
Do not be overwhelmed! Each of the relevant functional groups will be explained in detail in the next few topics.
For now it is important to be aware that compounds with the same functional groups will always end with the same suffix.
For example
- All alkanes will end with ‘___ane’
- All alkenes will end with ‘___ene’
- All alcohols will end with ‘___anol‘
- All carboxylic acids will end with ‘___anoic acid’
- All esters will end with ‘___yl ___anoate”
Naming compounds
Naming an organic compound can be done in 3 easy steps:
- Find the suffix (dictated by its functional group)
- Find the prefix (dictated by number of carbon atoms)
- Find the position of the functional group
We will use this compound below as an example. Let’s determine it’s name step by step:
Step 1: Find the suffix
This will eventually become easy for you as we dig into the details of various functional groups in future topics. You should eventually be able to look at any compound and state immediately which homologous series it belongs to.
The molecule above is an example of an alcohol because it contains the functional group -O-H.
The suffix of this molecule is therefore ‘___ol’
Step 2: Find the prefix
The prefix of organic molecules are strictly dependent on the number of carbon atoms present in the carbon chain of the molecule.
The example molecule contains 4 carbons in its chain so therefore the prefix is ‘but___‘.
Step 3: Find the position of the functional group
The position of the functional group must be specified in the name of the compound. This is simply the position of the carbon atom which is bonded to the functional group (-OH in this case).
In our example above, the OH functional group is attached to the second carbon on the carbon chain.
This position number goes between the prefix and suffix in the name of the compound.
It is important that you make sure that the functional group is closest to the beginning of the carbon chain. For example, I want you to imagine a chain of 5 carbons : C-C-C-C-C. Now counting from the LHS, imagine the OH functional group being attached to the fourth carbon on the chain. The position of the carbon is NOT four. It is in fact one since that would make the OH functional group closest to the beginning of the chain (counting from the LHS).
ANSWER:
Butan-2-ol