Definitions
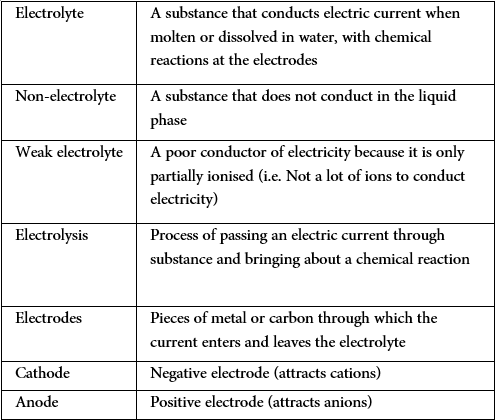
Electrolysis
Electrolysis is the breakdown of an ionic compound (molten or aqueous solution) by the passage of electricity.
Fundamentals
Reactions at the cathode or anode
Electrons flow from the battery to the cathode. Cations (usually metal and hydrogen ions) in the electrolyte are attracted to the cathode (negative electrode). Cations accepted electrons from the cathode, and therefore metals and hydrogen are formed at the cathode. For example:
Electrons flow from the anode to the battery. Negative ions (non-metals except hydrogen) are attracted to the anode (positive electrode).
- If the anode is inert (i.e. carbon or platinum) the negative ions lose electrons to the anode:
- If the anode is not inert (i.e. silver, copper, or other reactive metals) the metal atoms of the anode lose electrons and form positive ions. The anode will therefore dissolve and become smaller:
Ions of an electrolyte
The electrolyte can either be molten or aqueous.
- A molten substance means that the substance has been melted down. The ions therefore come only from the substance itself.
- An aqueous solution means that the substance is dissolved in water. The water molecules themselves can ionize so you will always find hydrogen and hydroxide ions in addition to the ions from the solute.
The discharge of ions
As we looked at above, ions are discharged at the anode or cathode.
In many cases, you will find that there are more than one cations or anions inside the electrolyte. For example:
- At the cathode you will find that the H+ will get discharged rather than Na+.
- At the anode you will find that OH- will get discharged rather than Cl-.
The electrochemical series tells us which ions discharge easier than others. The lower ion of each series will be the one to get discharged.
*Important note: In a concentrated solution, Cl- will be discharged rather than OH- despite what it says on the electrochemical series. In a dilute solution on the other hand, OH- will be discharged instead.
Important examples
Follow the basic principles for each example. Firstly, figure out the ions inside the electroyte. Secondly, figure out which ions will be discharged (from the eletrochemical series). Write down the reactions at the electrodes and also figure out what remains inside the final electrolyte.
Molten sodium chloride (inert electrodes)
- Ions present:
- Reactions in electrodes:
- Sodium chloride is therefore decomposed
Concentrated aqueous sodium chloride (inert electrodes)
- Ions present:
- Reactions in electrodes:
- Na+ and OH- remain in the electrolyte (which is sodium hydroxide)
*If the solution was dilute, then OH- would get discharged instead of the Cl-. This means Na+ and Cl- would remain in the electrolyte and the solution will become more and more concentrated (as water is used up).
Concentrated hydrochloric acic (inert electrodes)
- Ions present:
- Reactions in electrodes:
- Acid therefore gets used up in the electrolyte
Dilute sulfuric acid (inert electrodes)
- Ions present:
- Reactions in electrodes:
- Acid gets more concentrated as water gets used up
Aqueous copper (II) sulphate (Inert electrodes)
- Ions present:
- Reactions in electrodes:
- H+ and SO4 ions remain in the solution (which is sulfuric acid)
Aqueous copper (II) sulphate (copper electrodes)
- Ions present:
- The only difference is that the anode is not inert. This means that the metal anode itself will react by losing electrons to form ions.
- Copper deposited at the cathode becomes thicker. Copper is removed at the anode and it gets thinner. The electrolyte remains the same since one electrode produces copper ions whereas the other removes them. This process is used to electroplate other metals with copper.
Commercial use of electrolysis
Electroplating
This is used to plate one metal with another. The general arrangement for electroplating is shown here:
The metals commonly used to electroplate are copper, chromium, nickel, and silver. The two main reasons for electroplating are appearance and protection from corrosion.
Refining metals
Metals can be refined or purified by electrolysis. The impure metal forms the anode, the cathode is a small piece of pure metal and electrolyte is an aqueous metal salt. In the refining of copper, the following reactions occur
Cathode:
- Copper ions from solution lose their charge and copper is deposited
Anode:
Anode:
- Copper atoms lose their valency electrons and go into solution as ions
Overall pure copper is transferred from the anode to the cathode. The impurities from the copper are left as ‘anode slime’ and the cathode becomes a large piece of pure copper.
Aluminium extraction
Critical information:
- Main ore of aluminium is called bauxite
- It is changed to pure aluminium oxide (alumina)
- Graphite cathode and anode (therefore made of carbon)
- Electrolyte is molten mixture of pure aluminium oxide dissolved in cryolite
- The point in cryolite is to lower the temperature from approximately 2000 to 900 degrees.
- Reactions at electrodes:
- The carbon anodes burn away in oxygen and is replaced periodically
Industrial use of sodium chloride
As we looked at above, the use of concentrated sodium chloride can be used in electrolysis to make hydrogen gas, chlorine gas, and sodium hydroxide.
- Chlorine can be then used in making solvents, treating drinking water, bleach, etc.
- Hydrogen is used in the haber process, making fuels in cells, making margarine etc.
- Sodium hydroxide is used in soap manufacture
Electric cables: Conductors and insulators
Copper and aluminium are commonly used as conductors in electric cables. You need to know why they are good for this purpose.
- Copper
- Good conductor of electricity
- Ductile
- Easily purified
- Aluminium
- Good conductor
- Resists corrosion
- Low density, allowing high diameter cables to be used. This reduces resistance and sagging.
Plastics and ceramics are often used as insulators in electric cables.
- Plastics
- Do not conduct electricity
- Flexible & easily molded
- Non-biodegradable
- Ceramics
- Do not conduct electricity
- High melting points allowing use at high temperatures
- Not affected by water or oxygen
- Can be molded into complex shapes