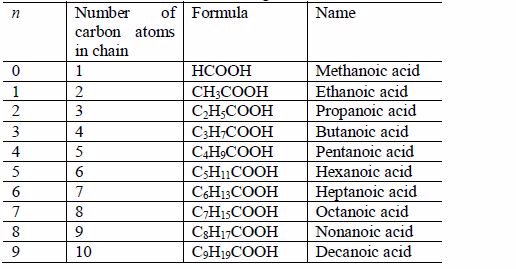
Carboxylic acids
All organic acids (carboxylic acids) have the general formula:
![]()
All of them also have the functional group -COOH and their names end in -oic acid.
Carboxylic acid homologous series
Ethanoic acid manufacture
Ethanoic acid is the second carboyxlic acid in the homologous series (look above). Ethanoic acid can be formed in two ways:
- Oxidation of ethanol via fermentation
- Oxidation of ethanol via acidified potassium manganate (VII)
Oxidation via fermentation
Acetobacter bacteria can oxidise (ferment) ethanol into ethanoic acid.
![]()
Oxidation via acidified potassium manganate (VII)
When ethanol is heated with the oxidising agent acidified potassium manganate (VII), ethanoic acid is formed.
![]()
Properties of aqueous ethanoic acid
All carboxylic acids (including ethanoic acid) is a weak acid. This means that they demonstrate typical acid properties (see chapter 8) and they only partially ionize in aqueous solution.
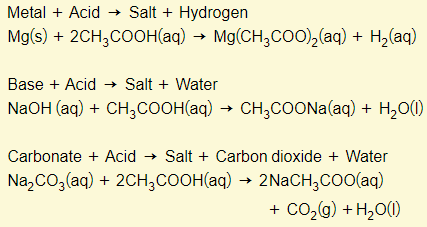
Formation of esters
Carboxylic acids react with alcohols to form an ester and water.
The conditions required for this reaction are:
- Heat
- Concentrated sulfuric acid catalyst
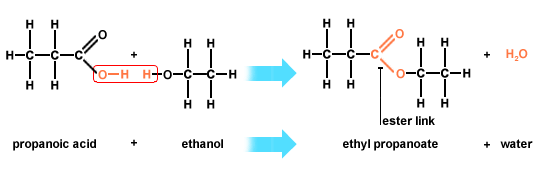
You can see that this is a condensation reaction (removal of water). The ‘OH’ from the -COOH functional group reacts with the ‘H’ from the -OH functional group to form water. The carbon (from -COOH) and oxygen (from -OH) join together to form the ester bond.
When naming the ester, the alcoholic portion always comes first (i.e. ethyl) and the carboxylic portion comes second (i.e. propanoate)