Homologous series
In organic chemistry, a homologous series is a series of compounds with the same functional group.
Due to the presence of the same functional group, compounds within the same homologous series have similar chemical properties.
The characteristics of a homologous series is outlined below:
- All members of the series can be presented by the same general formula
- Consecutive members of the series differ by CH2
- They have similar chemical properties because they have the same functional group
- Their physical properties change in a predictable way
The main functional groups that you need to be aware of are:
- Alkanes
- Alkenes
- Alcohols
- Carboxylic acids
Each of these functional groups and their unique reactions will be detailed within the next few topics.
Lets take a brief look at the homologous series of alkanes for example.
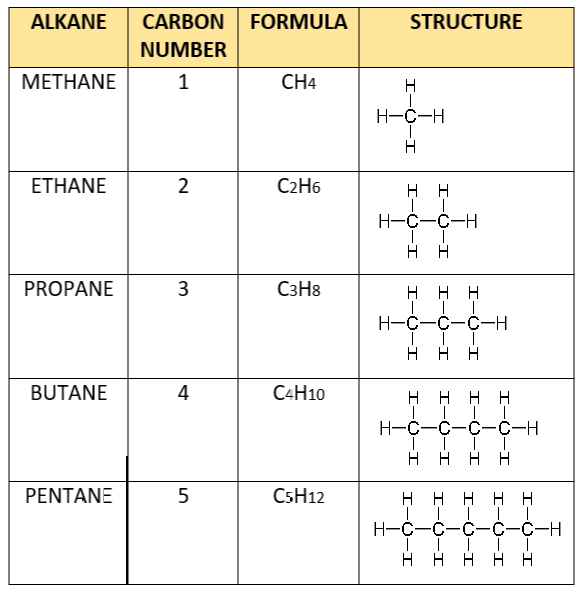
You can tell that all the molecules in the series (from methane to pentane) have the same general formula:
Furthermore, from methane to ethane and from ethane to propane (i.e. consecutive members of the series) – They all differ by CH2.
Structural isomerism
I strongly advise coming back to this section after covering alkanes, alkenes, alcohols, carboxylic acids, and polymers. It will make a lot more sense
Structural isomers are molecules with the same molecular formula but have a different structure. Despite the fact that they have the exact same number and types of atoms, they have a different structure due to the position of the functional group in the molecule.
The two molecules below are alcohols with the exact same molecular formula. However, they are clearly structurally different. This is because in the first molecule, the -OH functional group is on the 3rd carbon in the chain. In the second molecule however, the -OH group is on the 2nd carbon instead.